
Application Note
EarlyTox Caspase-3/7 NucView 488 Assay Kit on the SpectraMax i3x Multi-Mode Detection Platform
- Optimized for fluorescence microplate readers and fluorescence imaging
- Simple workflow with single reagent addition
- Optional Masking Reagent to reduce background fluorescence
Introduction
Apoptosis is an important mechanism that causes the programmed death of cells in normal processes such as embryonic development as well as diseases including cancer and neurodegenerative conditions. Assays for apoptosis can be performed using a variety of imaging or microplate readers and provide valuable information on normal and disease-related mechanisms of cell death.
The EarlyTox™ Caspase-3/7 NucView™ 488 Assay Kits enable detection of apoptosis in intact cell populations through use of NucView 488 Caspase-3 substrate. This substrate consists of a fluorogenic DNA dye coupled to the caspase-3/7 DEVD recognition sequence. Initially non-fluorescent, it permeates the cell membrane, and if the cell is apoptotic, the substrate is cleaved by caspase-3/7, releasing a dye that enters the nucleus and binds to DNA, resulting in bright green fluorescence.
Many fluorescence-based apoptosis assays are optimized for use only with microscopy or imaging systems, but the EarlyTox Caspase-3/7 NucView 488 Assay is optimized for both. Here we show how this flexible assay can be performed using either the SpectraMax® MiniMax™ 300 Imaging Cytometer or SpectraMax® i3x Multi-Mode Microplate Reader (Figure 1). Imaging data are easily analyzed using SoftMax® Pro Software, which also contains a preconfigured protocol for automatic calculation of plate reader results.
Figure 1: EarlyTox Caspase-3/7 NucView 488 Assay workflow.
Materials
- EarlyTox Caspase-3/7-D NucView 488 Assay Kit
- DMSO formulation
- *
- Explorer Kit (2-plate size, Molecular Devices P/N R8348)
- Bulk Kit (10-plate size, Molecular Devices P/N R8349)
- EarlyTox Caspase-3/7 NucView 488 Assay Kit
- PBS formulation
- Explorer Kit (2-plate size, Molecular Devices P/N R8350)
- Bulk Kit (10-plate size, Molecular Devices P/N R8351)
- PBS formulation
- HeLa cells (ATCC P/N CCL-2)
- Staurosporine (Sigma P/N S5921)
- 96-well black, clear-bottom microplates (Corning P/N 3904)
- SpectraMax i3x Multi-Mode Microplate Reader
- SpectraMax MiniMax 300 Imaging Cytometer
*Here, the NucView 488 with DMSO formulation was used.
Methods
HeLa cells were plated at 15,000 cells per well in 100 µL of medium in a 96-well black, clear-bottom microplate. They were allowed to attach and grow overnight in a 37°C, 5% CO2 incubator. They were then treated for 20 hours with a 1:2 dilution series of staurosporine from 5 µM down to 0.005 µM to induce apoptosis.
A 10 µM 2X working solution of NucView 488 substrate was prepared in culture medium. 100 µL of working substrate solution was added directly to wells containing 100 µL of cells and medium for a final concentration of 5 µM. Cells were incubated at room temperature for 15-30 minutes, protected from light. Imaging was performed on a SpectraMax MiniMax 300 Imaging Cytometer using the 541 nm green fluorescence channel, or detected on the SpectraMax i3x Multi-Mode Microplate Reader using an excitation wavelength of 490 nm and emission wavelength of 535 nm.
For assays detected on a microplate reader, an optional Masking Reagent provided in the kit was added to cells after the substrate incubation was completed. Masking Reagent was added to cells at the recommended dilution for the kit, typically a 1:4 dilution of the reconstituted vial for an Explorer kit. After addition of Masking Reagent, the plate was immediately read from the bottom on the plate reader.
Results
HeLa cells treated with staurosporine exhibited an apoptotic response that could be detected and quantified using the EarlyTox Caspase-3/7 NucView 488 Assay. Fluorescently-labeled apoptotic cells were detected using the SpectraMax i3x Multi-Mode Microplate Reader and the SpectraMax MiniMax 300 Imaging Cytometer
Apoptotic cells imaged on the SpectraMax MiniMax 300 Imaging Cytometer (Figure 2) were identified and analyzed using SoftMax Pro Software. Graphing the apoptotic cell counts vs. staurosporine concentration and applying a 4-parameter curve fit resulted in a concentration response curve with EC50 value of 0.10 µM (Figure 3).
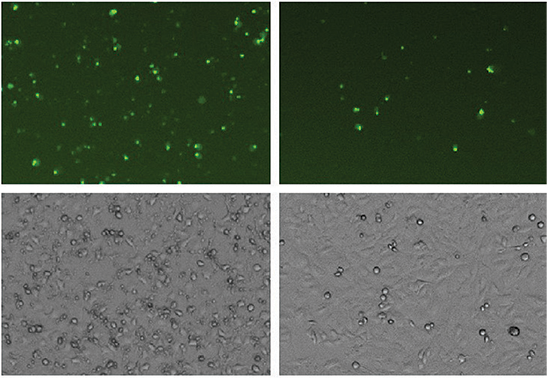
Figure 2: HeLa cells treated with staurosporine for 20 hours to induce apoptosis. NucView 488 substrate was added at a final concentration of 5 µM. Cells were imaged using the green (541 nm) fluorescence channel (top) and transmitted light channel (bottom) of the SpectraMax MiniMax 300 Imaging Cytometer.
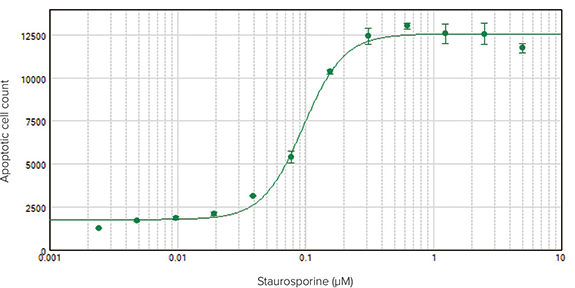
Figure 3. Concentration-response curve for staurosporine-treated HeLa cells imaged on the SpectraMax MiniMax 300 Imaging Cytometer. Apoptotic (green fluorescent) cells were identified in the images and counted using SoftMax Pro Software. EC50 = 0.10 µM.
Assays detected on the SpectraMax i3x Multi-Mode Microplate Reader also showed a concentration-dependent response of HeLa cells to staurosporine (Figure 4, top). Because plate readers detect total fluorescence per well rather than individual cells against a background of fluorescence, the resulting curve does not reach a plateau. However, addition of Masking Reagent to the wells just prior to reading the plate reduced background values, resulting in a complete 4-parameter concentration response curve with EC50 value of 0.32 µM, similar to that obtained via imaging (Figure 4, bottom).
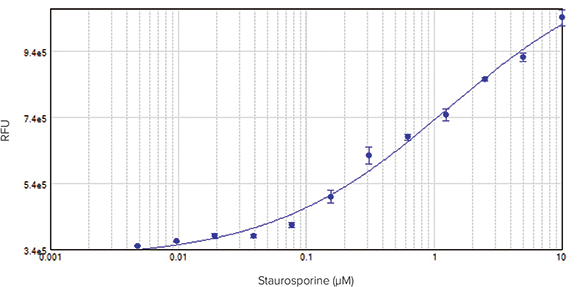
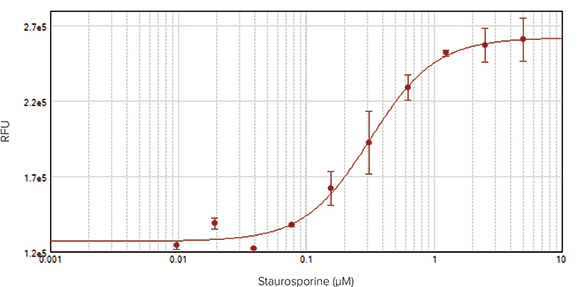
Figure 4: HeLa cells treated with staurosporine for 20 hours to induce apoptosis. NucView 488 substrate (DMSO formulation) was added at a final concentration of 5 µM. Fluorescence corresponding to caspase activity was measured using a SpectraMax i3x Multi-Mode Microplate Reader.Top: Assay without Masking Reagent. Bottom: Assay with Masking Reagent added to wells to reduce background (EC50 = 0.32 µM).
Conclusion
The EarlyTox Caspase-3/7 NucView 488 Assay Kit, used together with either SpectraMax microplate readers or SpectraMax MiniMax 300 Imaging Cytometer, provides users with a flexible approach to the quantitation of apoptosis. Apoptotic cells can be measured directly as a function of total fluorescence per well, or by imaging and counting individual cells, with comparable results for both methods. An optional Masking Reagent reduces background fluorescence and enables more accurate calculation of EC50 values when this information is required. The NucView 488 substrate is available in two formulations: DMSO and PBS. The PBS formulation may be used for cells that are sensitive to DMSO.