
Application Note
Qualitative measurement of SARS-CoV-2 spike and nucleocapsid IgG antibodies in serum samples
- Gain >95% specificity for nucleocapsid and spike IgG antibodies
- Measure up to 86 samples per kit with high-throughput, qualitative immunoassays
- Acquire colorimetric readout with exceptional inter/intra assay precision
Cathy Olsen, PhD | Sr. Applications Scientist | Molecular Devices
Heather Mary Brown, PhD | Application Scientist | Enzo Life Sciences
Introduction
Rapid, accurate, and frequent molecular testing is of critical importance in navigating the SARS-CoV-2 (COVID-19) pandemic. To advance our understanding of the relationship between infection and immunity, it is critical to test not only for infection, but also for antibodies. The fundamental challenge is to identify and implement sensitive, consistent, and accessible testing methods that fulfill these requirements, all at an affordable price. Quantitative RT-PCR testing for detecting viral mRNA is currently the most accurate test on the market for identifying an existing infection. However, it does not shed light on the immune response. Given that significant emphasis has been placed on developing testing methods to identify disease, advancements in assay development for antibody testing have remained insufficient. The antibody tests currently on the market return many false positive results and therefore are not a reliable method for understanding the nuances of the immune response to SARS-CoV-21. Thus, a reliable, high-throughput method for investigating COVID-19 immunity and antibody response is an unmet need.
Enzo has developed a state-of-the-art enzyme linked immunosorbent assay (ELISA) that is specifically optimized to detect IgG antibodies against the SARS-CoV-2 Nucleocapsid protein in human serum and plasma samples (ENZ-KIT193). SARS-CoV-2 Nucleocapsid IgG protein is an antibody generated as part of the adaptive human immune response to the Nucleocapsid protein of the SARS-CoV-2 virus. The Nucleocapsid protein is important for RNA packaging and virus particle release. The presence of antibodies against the Nucleocapsid protein indicate a recent or prior infection. The Nucleocapsid protein is highly conserved and is less susceptible to mutations over time, making this an optimal target for long-term studies of COVID-19 immunity2.
Additionally, Enzo has developed a complimentary ELISA kit, SARS-CoV-2 Spike IgG ELISA Kit (RUO) (ENZ-KIT190), enabling detection of specific antibodies against the SARS-CoV-2 Spike protein in human serum. SARS-CoV-2 Spike IgG protein is another antibody generated via the adaptive human immune response to the Spike protein of the SARS-CoV-2 virus. Spike protein is essential for binding of the virus to the ACE2 receptor on the surface of human cells for entry into the cell for replication. Thus, it is a target for some vaccines currently available.
Enzo’s SARS-CoV-2 Nucleocapsid IgG ELISA Kit and SARS-CoV-2 Spike IgG ELISA Kit are keystone tools for current and future studies to determine antibody response and immunity to COVID-19 infection and vaccine studies.
Both ELISA kits exhibit a broad detection range, high sensitivity, and >95% specificity for their target analytes, Nucleocapsid IgG antibody and Spike IgG antibody, in up to 86 samples within 30 minutes or two hours, respectively. Thus, these kits exceed requirements for accuracy and speed, and offer the potential for many applications to propel research forward. Developing a deeper understanding of the antibody behavior post-virus exposure will allow researchers to reveal characteristics of SARS-CoV-2 immune response following infection, and also serve as a tool for understanding the fundamental questions regarding immunity to this highly infectious virus.
Materials
- SARS-CoV-2 Spike IgG ELISA Kit (RUO) (Enzo cat. #ENZ-KIT190-0001)
- SARS-CoV-2 Nucleocapsid IgG ELISA Kit (RUO) (Enzo cat. #ENZ-KIT193-0001)
- Human Serum COVID-19 Positive (BioIVT cat. #HMSRM-COVIDIGG):\
- Lot #HMN368394-SR1
- Lot #HMN368436-SR1
- Lot #HMN368437-SR1
- Lot #HMN373782-SR1
- Lot #HMN373791-SR1
- Lot #HMN374206-SR1
- Human serum, off the clot (negative) (Amsbio cat. #HSER-2mL):\
- Lot #122019A
- SpectraMax® ABS Plus Microplate Reader
Methods
Assay setup
Materials from each of the two kits were brought to room temperature prior to use, including well strips sufficient to run assay controls and duplicate serum samples for each kit. 1X Wash Buffer was prepared by diluting the 20X Wash Buffer Concentrate with water.
Serum samples from patients who had tested positive for SARS-CoV-2, or normal serum collected pre-pandemic, were prepared by diluting them in Sample Diluent as indicated in the product manual for each kit.
The steps for running each ELISA kit are shown in Table 1.
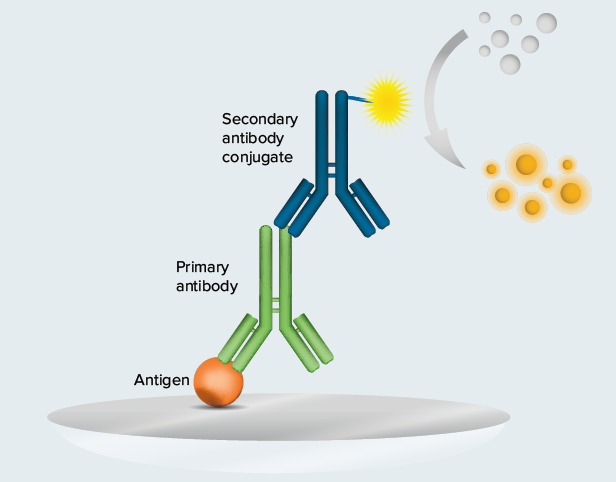
Figure 1. SARS-CoV-2 IgG ELISA (indirect ELISA format). SARS-CoV-2 IgG ELISAs schematic. The bottom of the wells are coated with SARS-CoV-2 Nucleocapsid protein or Spike S1-receptor binding domain (antigen). If present in the serum sample, IgG specific for the antigen binds to the coated wells, and an HRP-conjugated antibody enables detection of the bound IgG with the addition of substrate.
Table 1. Steps for running each SARS-CoV-2 ELISA Kit.
Calculation of results for SARS-CoV-2 Nucleocapsid IgG ELISA
Net OD values were calculated by subtracting the average OD value of the blank wells from all OD values for all wells, including the calibrators and serum samples.
Net Sample OD = Sample OD - Average Blank OD
The average OD value for each calibrator was plotted vs. its concentration using a 4-parameter logistic curve fit in SoftMax® Pro Software (Figure 2).
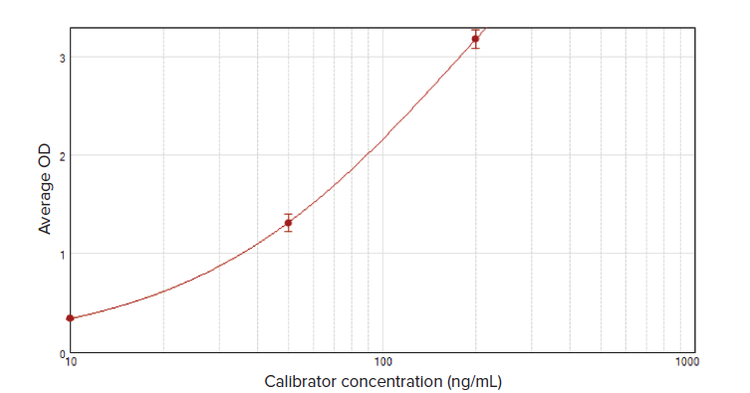
Figure 2. Calibrator curve for the SARS-CoV-2 Nucleocapsid IgG ELISA Kit. The curve was plotted in SoftMax Pro Software using a 4-parameter logistic curve fit. From this curve, results were calculated semi-quantitatively.
Results were then calculated semi-quantitatively by interpolation off the calibrator curve. An interpolated concentration ≤ 2000 ng/mL implied the absence of SARS-CoV-2 IgG, while an interpolated concentration > 2000 ng/mL implied the presence of SARS-CoV-2 IgG.
Calculation of results for SARS-CoV-2 Spike IgG ELISA
Net OD values were calculated by subtracting the average OD value of the blank wells from all OD values for all wells, including the Negative Control, Low Positive Control, High Positive Control, and serum samples:
Net Sample OD = Sample OD - Average Blank OD
An Index Value was calculated for each sample using the following formula:

A cut-off value of 0.08 was given in the product manual and used to calculate results. Index values ≥ 1 indicated a positive result, while index values < 1 indicated a negative result. All calculations were set up in group tables in SoftMax Pro Software, enabling automatic calculation of results when the ELISA plate was read.
Results
For both ELISA kits, the serum samples from COVID-19 positive patients tested positive, meaning that all of these samples contained both nucleocapsid IgG and spike IgG. The normal serum sample tested negative for both assays. Group tables from SoftMax Pro Software showing results automatically calculated from the data are shown in Table 2 (Nucleocapsid IgG ELISA kit) and Table 3 (Spike IgG ELISA kit).
C2
D2
0.401
0.328
12.201
9.645
E2
F2
3.449
3.488
239.379
245.870
G2
H2
2.192
1.991
102.831
88.732
A3
B3
2.144
2.266
99.288
108.329
C3
D3
2.160
2.149
100.476
99.704
E3
F3
0.594
0.547
19.202
17.461
G3
H3
0.066
0.055
0.923
0.564
Table 2. Results of SARS-CoV-2 Nucleocapsid IgG ELISA Kit. Results were calculated semi-quantitatively via interpolation from the calibrator curve and displayed in a group table in SoftMax Pro Software. ‘R’ indicates samples with OD values that fell outside the range of the calibrator curve.
C2
D2
1.771
1.784
E2
F2
2.100
2.024
G2
H2
2.508
2.418
A3
B3
1.972
1.990
C3
D3
1.869
1.804
E3
F3
0.790
0.766
G3
H3
0.039
0.028
Table 3. Results of SARS-CoV-2 Spike IgG ELISA Kit. Index values were calculated and sample results were displayed as positive or negative in a group table in SoftMax Pro Software
Conclusion
Overall, these data show that the Enzo kits, SARS-CoV-2 Nucleocapsid IgG ELISA Kit (RUO) (ENZ-KIT193) and SARSCoV- 2 Spike IgG ELISA Kit (RUO) (ENZ-KIT190), are an accurate, high-throughput and rapid solution for the detection of Nucleocapsid IgG antibodies and Spike IgG antibodies in human samples. Furthermore, these kits are easily run on the SpectraMax ABS Plus reader with SoftMax Pro Software, which enable consistent and efficient generation of results. These quantitative and semi-quantitative data provide valuable metrics for specific IgG analysis for an individual patient sample as well as accurate data to contribute to population surveillance studies. Thus, they present novel opportunities for implementation of these solutions in the clinic for future investigations into understanding the nuances of the immunogenic response, duration of immunity, and patterns in population studies of infection and immunity response to SARS-CoV-2. The applications for both kits can play a major role in the prevention and management of future pandemics.
References
- https://www.uclahealth.org/antibody-serology-testing#howdoestestwork
- Dutta NK, Mazumdar K, Gordy JT. The Nucleocapsid Protein of SARSCoV-2: a Target for Vaccine Development. J Virol. 2020;94(13):e00647-20. Published 2020 Jun 16. doi:10.1128/JVI.00647-20